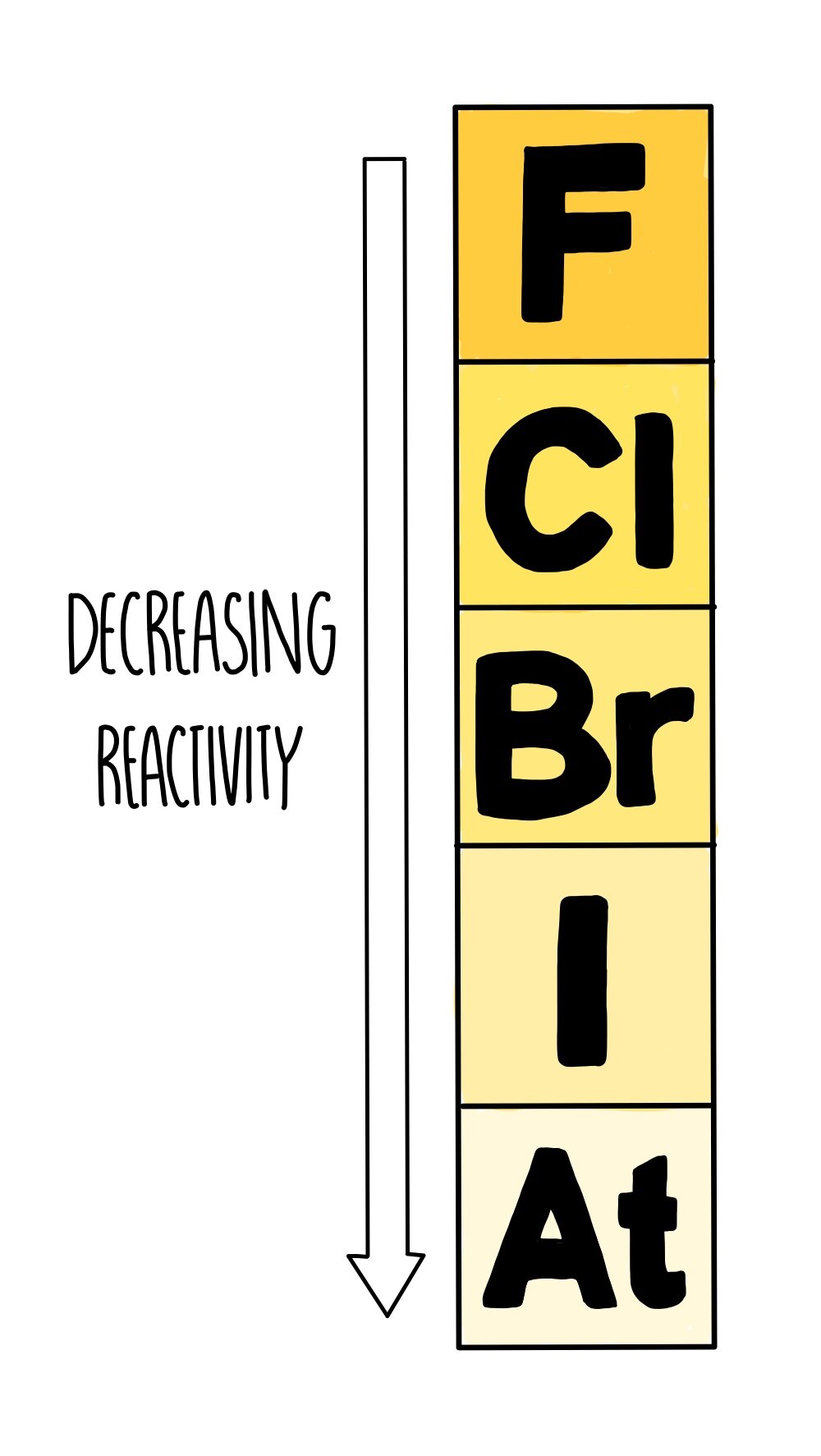
Property Of Halogens Reactivity . The group 7 elements are also known as the halogens. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. The three common group 7 elements are. Their main property is reactivity. Group 7 halogens reactivity of halogens. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. These reactive nonmetals have seven valence electrons. All halogens form group 1 salts with similar. All of the halogens are nonmetals. As a group, halogens exhibit highly variable physical properties. As the atomic number increases, the reactivity of the halogens. The halogens all have seven electrons in their outer shells. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. Halogens are reactive because they want to obtain that last electron to fill their outer level.
from www.thesciencehive.co.uk
As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. As the atomic number increases, the reactivity of the halogens. These reactive nonmetals have seven valence electrons. Halogens are reactive because they want to obtain that last electron to fill their outer level. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. All halogens form group 1 salts with similar. Group 7 halogens reactivity of halogens. Their main property is reactivity. The group 7 elements are also known as the halogens. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room.
The Halogens* — the science sauce
Property Of Halogens Reactivity The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. The halogens all have seven electrons in their outer shells. The three common group 7 elements are. Their main property is reactivity. Group 7 halogens reactivity of halogens. Halogens are reactive because they want to obtain that last electron to fill their outer level. As a group, halogens exhibit highly variable physical properties. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. As the atomic number increases, the reactivity of the halogens. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. All halogens form group 1 salts with similar. These reactive nonmetals have seven valence electrons. The group 7 elements are also known as the halogens. All of the halogens are nonmetals.
From www.tes.com
Lesson Halogen Displacement GCSE Edexcel 91 Teaching Resources Property Of Halogens Reactivity The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. These reactive nonmetals have seven valence electrons. All halogens form group 1 salts with similar. Their main property is reactivity. The halogens all have seven electrons in their outer. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Property Of Halogens Reactivity The electron configuration in the outer shell is ns2np5 n s 2 n p 5. Halogens are reactive because they want to obtain that last electron to fill their outer level. These reactive nonmetals have seven valence electrons. All of the halogens are nonmetals. As the atomic number increases, the reactivity of the halogens. Group 7 halogens reactivity of halogens.. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Lecture 16. The Halogens PowerPoint Presentation, free download Property Of Halogens Reactivity Halogens are reactive because they want to obtain that last electron to fill their outer level. Their main property is reactivity. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. The halogens all have seven electrons in their. Property Of Halogens Reactivity.
From scienceinfo.com
Chemical Properties of Halogen Elements and Hydrogen Halides Property Of Halogens Reactivity As the atomic number increases, the reactivity of the halogens. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. All of the halogens are nonmetals. All halogens form group 1 salts with similar. The electron configuration in the. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Property Of Halogens Reactivity These reactive nonmetals have seven valence electrons. The group 7 elements are also known as the halogens. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. As the atomic number increases, the reactivity of the halogens. Group 7 halogens reactivity of halogens. Their main property is reactivity. The group of halogens is the only. Property Of Halogens Reactivity.
From www.thesciencehive.co.uk
Group 7 (Halogens) (GCSE) — the science sauce Property Of Halogens Reactivity The three common group 7 elements are. Their main property is reactivity. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The halogens all have seven electrons in their outer shells. As a group, halogens exhibit highly variable physical properties. As the atomic number increases, the reactivity of the halogens. Halogens are reactive because. Property Of Halogens Reactivity.
From www.youtube.com
Explaining the Halogen Reactivity Trend and other properties of group 7 Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. As the atomic number increases, the reactivity of the halogens. As a group, halogens exhibit highly variable physical properties. Group 7 halogens reactivity of halogens. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The three common group. Property Of Halogens Reactivity.
From warreninstitute.org
Exploring The Chemical Reactivity Of Halogens. Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. The group 7 elements are also known as the halogens. As the atomic number increases, the reactivity of the halogens. These reactive nonmetals have seven valence electrons. All halogens form. Property Of Halogens Reactivity.
From study.com
Halogens of the Periodic Table Properties, Reactivity & Uses Lesson Property Of Halogens Reactivity All halogens form group 1 salts with similar. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. As a group, halogens exhibit highly variable physical properties. These reactive nonmetals have seven valence electrons. Halogens are reactive because they want to obtain that last electron to fill their outer level. Their main property is reactivity.. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Property Of Halogens Reactivity All of the halogens are nonmetals. These reactive nonmetals have seven valence electrons. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. All. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. Their main property is reactivity. As the atomic number increases, the reactivity of the. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Reactions of the halogens and halide ions PowerPoint Presentation Property Of Halogens Reactivity The halogens all have seven electrons in their outer shells. All of the halogens are nonmetals. Group 7 halogens reactivity of halogens. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. These reactive nonmetals have seven valence electrons. Halogens are reactive because they want to obtain that last electron to fill their outer level.. Property Of Halogens Reactivity.
From quizlet.com
Reactivity trends (Group 17 the halogens) Diagram Quizlet Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. Their main property is reactivity. As the atomic number increases, the reactivity of the halogens. The halogens all have seven electrons in their outer shells. Group 7 halogens reactivity of halogens. As a general rule, fluorine is the most reactive halogen and astatine is the. Property Of Halogens Reactivity.
From www.tes.com
Halogen Displacement Reactions GCSE AQA Teaching Resources Property Of Halogens Reactivity As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The three common group 7 elements are. Their main property is reactivity. The group 7 elements are also known as the halogens. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The group of halogens is the only. Property Of Halogens Reactivity.
From utedzz.blogspot.com
Periodic Table Trends Reactivity Periodic Table Timeline Property Of Halogens Reactivity The three common group 7 elements are. Halogens are reactive because they want to obtain that last electron to fill their outer level. The electron configuration in the outer shell is ns2np5 n s 2 n p 5. Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. Group 7 halogens reactivity of halogens. As. Property Of Halogens Reactivity.
From utedzz.blogspot.com
Periodic Table Reactivity Periodic Table Timeline Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. The electron configuration in the outer shell is ns2np5 n s 2 n p. Property Of Halogens Reactivity.
From www.thesciencehive.co.uk
The Halogens* — the science sauce Property Of Halogens Reactivity Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. As a group, halogens exhibit highly variable physical properties. As the atomic number increases, the reactivity of the halogens. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure,. Property Of Halogens Reactivity.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download Property Of Halogens Reactivity The halogens all have seven electrons in their outer shells. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room. As the atomic number increases, the reactivity of the halogens. Their main property is reactivity. All of the halogens. Property Of Halogens Reactivity.